
The CLIA of 1988 established a federal framework regulating laboratory testing on human specimens for health purposes. For healthcare facilities like physician’s offices, urgent care centers, and others, understanding CLIA requirements is crucial for both regulatory compliance and ensuring proper medical billing and reimbursement.
The Clinical Laboratory Improvement Amendments (CLIA) were established in 1988 to ensure quality laboratory testing across the United States. Regulated by the Centers for Medicare & Medicaid Services (CMS), CLIA applies to all facilities that perform tests on human specimens for health assessment or to diagnose, prevent, or treat disease.
There are several types of CLIA certificates based on the complexity of testing performed:
Certificate of Waiver (CoW) – For laboratories performing only waived tests
Certificate for Provider-Performed Microscopy Procedures (PPMP) – For labs performing waived tests plus specific microscopy procedures
Certificate of Compliance (CoC) – For labs performing moderate and/or high complexity tests that undergo routine inspections
Certificate of Accreditation (CoA) – For labs performing moderate and/or high complexity tests accredited by an approved organization
Certificate of Registration (CoR) – A temporary certificate issued until determinations of compliance can be made
For many medical practices, the Certificate of Waiver is the most common and straightforward option.
To qualify for a CLIA waiver, your facility must:
Perform only waived tests that are simple with minimal risk of error
Follow manufacturers’ instructions for all waived tests
Pay the applicable certificate fee
Allow on-site inspections when requested by CMS
Notify your state agency about changes in ownership, name, address, or test menu
Maintain records of testing for at least two years
Obtaining a Certificate of Waiver involves these steps:
Complete Form CMS-116: The “Clinical Laboratory Improvement Amendments (CLIA) Application for Certification”
Submit to State Agency: Send the completed application to your state’s Department of Health CLIA program
Pay the Fee: The current fee for a Certificate of Waiver is $180 for two years
Receive Your Certificate: Once approved, you’ll receive a certificate valid for two years
The application requires information about your facility, director, ownership, testing personnel, and the specific tests you’ll perform.
A CLIA number (also called a CLIA ID) is a unique 10-character identifier assigned to your laboratory upon certification. This number is crucial for medical billing purposes as it must be included on claims for laboratory services submitted to Medicare and many other insurers.
The format typically follows this pattern:
First two characters: State code
Next two digits: Number representing the certificate type
Remaining characters: Unique identifier for the laboratory
The FDA maintains an updated list of CLIA-waived tests. Some common waived tests include:
Blood glucose monitoring
Hemoglobin A1C
Urine pregnancy tests
Rapid strep tests
Fecal occult blood tests
Influenza A/B rapid tests
COVID-19 rapid antigen tests
Cholesterol screening
Prothrombin time/INR
H. pylori antibody tests
The full list is extensive and continually updated as new tests receive waived status. The CDC maintains a comprehensive document listing all tests granted waived status, which can be accessed through their website.
When billing for CLIA-waived tests, you must use the appropriate CPT codes along with the QW modifier, which specifically indicates the test is CLIA-waived. Some common CLIA-waived test CPT codes include:
82962-QW: Glucose monitoring by glucose meter
83036-QW: Hemoglobin A1C test
81002-QW: Urinalysis, non-automated without microscopy
87880-QW: Strep A, direct optical observation
85610-QW: Prothrombin time (PT)
82270-QW: Blood occult, feces screening
It’s important to note that without the QW modifier, many claims for these laboratory tests will be denied by Medicare and other payers.
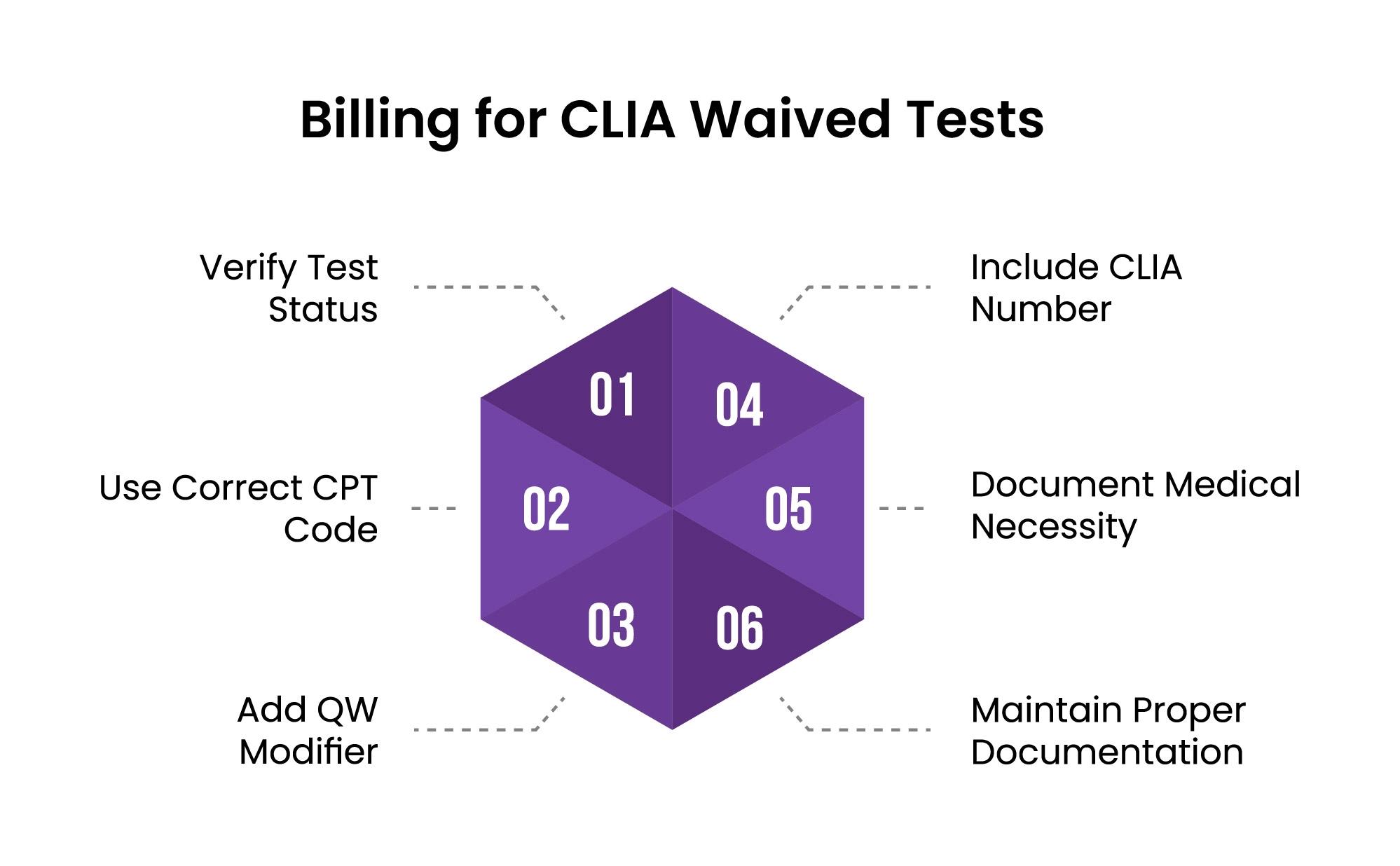
Before billing for any laboratory test as CLIA-waived, you must confirm its official classification on the FDA’s CLIA waived test list. This verification is critical because:
Test classifications can change over time as manufacturers update methodologies
Some tests may appear similar but have different complexity classifications
Performing or billing for tests beyond your certificate’s scope violates CLIA regulations
You can verify a test’s waived status through:
The FDA’s CLIA Database (accessible via their website)
The test manufacturer’s package insert which explicitly states the CLIA classification
The CDC’s comprehensive list of tests granted waived status (updated quarterly)
If a test is not officially classified as waived, billing it with a QW modifier would constitute improper billing and could trigger an audit.
Each laboratory test has specific CPT (Current Procedural Terminology) codes assigned by the American Medical Association that identify the precise procedure performed:
CPT codes are five-digit numeric codes (e.g., 82962 for glucose blood testing)
Tests may have multiple CPT code options depending on methodology used
The correct code must reflect the exact methodology of the test performed
Using an incorrect or less specific code may result in improper reimbursement or denials
For example, a rapid strep test might use code 87880 (Streptococcus, group A, direct optical observation), but if you performed a culture instead, a different code would be required, even though both test for strep.
The QW modifier is a critical two-character code specifically created by CMS to identify CLIA-waived tests:
It must be appended directly to the CPT code (e.g., 87880-QW)
The modifier tells the payer that:
The test is officially classified as CLIA-waived
Your facility is appropriately certified to perform it
The simplified methodology justifies a potentially different reimbursement rate
Without the QW modifier, claims processing systems may:
Automatically reject the claim
Flag it for manual review, delaying payment
Apply incorrect reimbursement rates
Medicare and many commercial payers will automatically deny laboratory claims for waived tests if the QW modifier is missing, even if the facility has the appropriate CLIA certificate.
Your CLIA identification number is a mandatory identifier that proves your laboratory’s certification status:
For paper claims using CMS-1500 forms:
Enter the 10-digit CLIA number in Box 23 (labeled “Prior Authorization Number”)
The entire number must be clearly legible
If multiple laboratories performed different tests on the same claim, you may need to submit separate claim forms
For electronic claims (ANSI X12 837P format):
Include in Loop 2300, REF segment
REF01 = “X4” (CLIA indicator)
REF02 = your 10-digit CLIA number
Your clearinghouse or billing software vendor can provide specific instructions for your system
Common claim rejection reasons related to CLIA numbers include:
Missing CLIA number
Expired certificate at time of service
Mismatch between CLIA number and performing provider
Certificate type doesn’t cover the procedure billed
Medical necessity documentation creates the clinical justification for performing the test:
Each test must be linked to a specific ICD-10 diagnosis code that supports the need for testing
The diagnosis must demonstrate why the test was reasonable and necessary
Documentation should include:
Patient symptoms or condition requiring diagnosis/monitoring
How test results will influence treatment decisions
Compliance with frequency limitations (some tests have restrictions on how often they can be performed)
Medicare and other payers maintain lists of “covered diagnoses” for certain laboratory tests through National Coverage Determinations (NCDs) and Local Coverage Determinations (LCDs). For example:
HbA1C testing (83036-QW) requires diabetes-related diagnosis codes
Lipid panel testing (80061-QW) requires cardiovascular risk or monitoring diagnoses
Pregnancy testing requires symptoms or conditions warranting confirmation of pregnancy
Comprehensive documentation is essential both for compliance and in case of audit:
Test requisition documentation should include:
Specific test ordered (not just “labs”)
Ordering provider’s signature
Date of order
Clinical indication/diagnosis
Testing documentation should include:
Date and time of specimen collection
Date and time of test performance
Identity of person performing the test
Test methodology used
Quality control results
Test results with appropriate reference ranges
Result interpretation when applicable
Additional records to maintain:
Equipment maintenance logs
Temperature logs for reagent storage
Staff training documentation
Manufacturer’s package inserts for all test systems
Lot numbers and expiration dates of testing supplies
Proper documentation serves multiple purposes:
Demonstrates regulatory compliance with CLIA requirements
Supports the medical necessity of testing for reimbursement
Provides evidence in case of payer audits
Creates legal protection in case of disputed results
Facilitates quality improvement initiatives
Healthcare providers must maintain ongoing CLIA compliance by:
Performing Only Authorized Tests: Stay within the scope of your certificate
Following Test Instructions: Adhere to manufacturers’ guidelines precisely
Training Personnel: Ensure all staff are properly trained
Quality Control: Implement quality control procedures as required
Certificate Renewal: Renew your CLIA certificate every two years
Notifying Changes: Report any significant changes in your facility or testing menu
Documentation: Maintain thorough records of all testing procedures, results, and quality control measures
Physician offices face specific CLIA considerations:
Provider-Performed Microscopy: Physicians who want to perform certain microscopy procedures need a PPMP certificate instead of a basic waiver
Multiple Locations: Each physical location where testing occurs needs its own CLIA certificate
Medical Director: A qualified physician must serve as the laboratory director
Staff Competency: Regular assessment of staff competency is required
Test Selection: Carefully consider which tests to perform in-house versus sending to reference labs
CLIA waivers represent an important regulatory framework that ensures quality laboratory testing while allowing healthcare providers to perform simple diagnostic tests in-house. Understanding the requirements for obtaining and maintaining a CLIA Certificate of Waiver—along with proper billing procedures—is essential for healthcare practices seeking to optimize their laboratory services.
By following the guidelines outlined in this article, you can ensure your practice remains compliant with CLIA regulations while maximizing appropriate reimbursement for the laboratory services you provide.
BillongLoop USA LLC is a leading healthcare services provider, managing numerous medical practices with compassionate, skilled professionals utilizing the latest tools and techniques. Our goal is to maintain the highest standards by providing our clients with complete RCM solutions.
This website uses cookies to provide you with the best browsing experience.